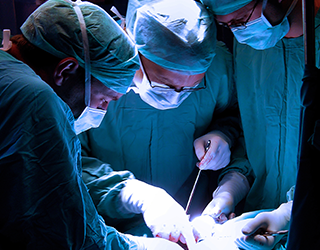
Medical Products and Systems
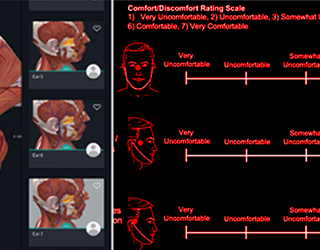
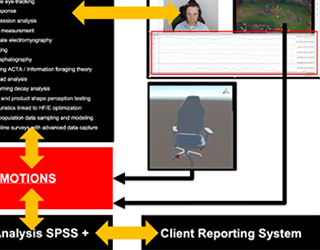
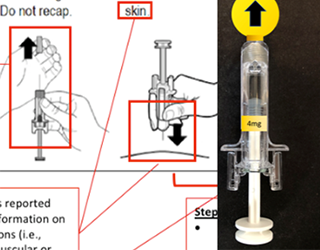
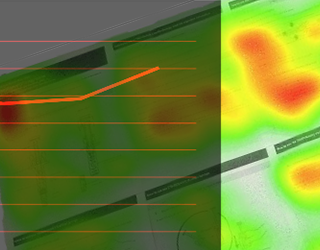
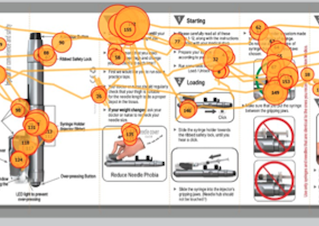
01In the medical products space we offer clients an exceptionally deep and broad level of experience focusing on FDA HF guidance and usability testing. We have developed, validated and utilized in business-critical medical device programs the most advanced testing methods in industry. We are experts in the application of FDA, ISO and other human factors optimization issues.